MSF applauds Indian Patent Office’s rejection of Johnson & Johnson’s attempt to extend monopoly on lifesaving TB drug

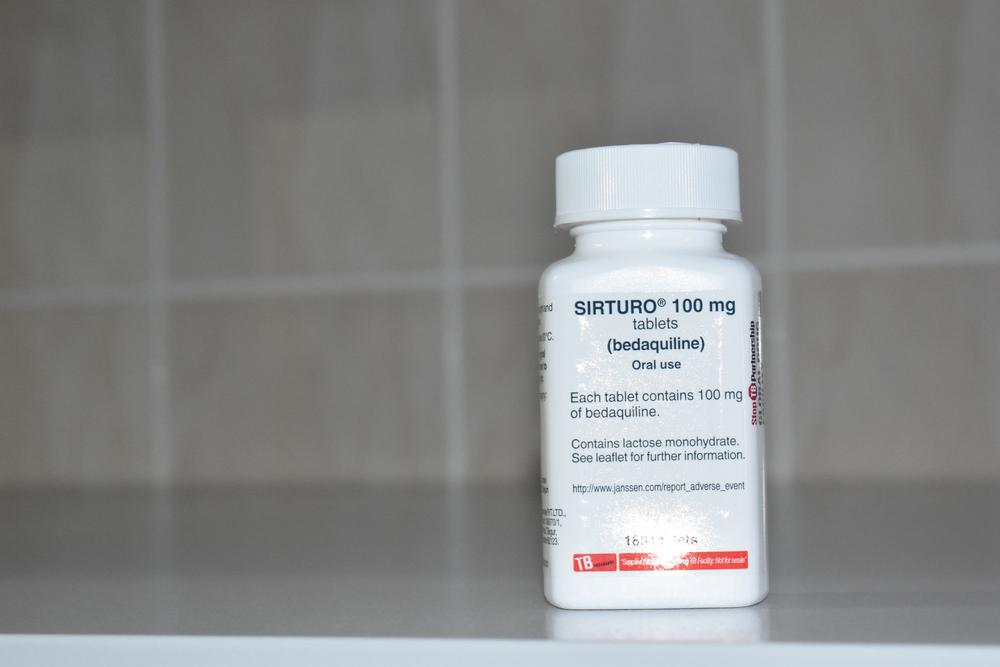
On March 2023, the 2019 patent challenge by two tuberculosis (TB) survivors, Nandita Venkatesan and Phumeza Tisile, was successful: the Indian Patent Office rejected the US pharmaceutical corporation Johnson & Johnson’s (J&J) attempt to extend its monopoly in India on the TB drug bedaquiline beyond the primary patent’s expiry this July. Médecins Sans Frontières/Doctors Without Borders (MSF) welcomed this as a significant step toward increasing access to the lifesaving TB drug.
Indian patent law does not allow the evergreening of patents and prevents pharmaceutical corporations from abusing the patent system through making minor changes that can further extend their 20-year drug monopolies. Such evergreening practises are explicitly prohibited in the Indian patent system through a health safeguard—Section 3(d). In South Africa, this monopoly-extending patent has been granted, and it prevents generic manufacturers from providing more affordable versions of bedaquiline for four more years, until 2027. Although South Africa is in the process of reforming its patent law, it must expedite the process and closely scrutinise all patents to remove barriers that may hinder access to medical products.
In the meantime, J&J must not block the supply of more affordable generic versions of bedaquiline to high TB burden countries like South Africa and stand by its 2019 statement from the Managing Director in India of Janssen (J&J’s pharmaceutical division), that generic manufacturers will be able to make generic versions of bedaquiline starting in 2023. This means that the US corporation should also urgently withdraw its patents in other countries, including high TB burden countries, where the equivalent of the Indian patent application have been granted, and are a barrier to generic supply.
Phumeza Tisile, extensively drug-resistant (XDR) TB survivor and petitioner in the patent opposition case, Cape Town, South Africa:
“My fellow TB survivor Nandita Venkatesan from India and I filed a patent challenge in India in 2019 to ensure price-lowering generic supply of lifesaving TB drug bedaquiline starts as soon as the basic patent expires on it in July 2023. The landmark rejection of J&J’s secondary patent on bedaquiline in India is a huge victory for people with drug-resistant TB in India and beyond! This shows that with progressive patent laws incorporating health safeguards, people are empowered to challenge unmerited monopolies and prevent the grant of unwarranted patents.”
“In the absence of a stricter patent examination process, this secondary patent was granted in South Africa thereby extending J&J’s monopoly on bedaquiline until 2027. We hope that today’s decision by the Indian Patent Office encourages the South African government to expeditiously finalise the reform of its patent laws. South Africa cannot afford to wait for a conclusion of the TRIPS Waiver and the African Continental Free Trade Agreement process (as recently said in parliament by the minister of Trade and Industry) before it finalises the reform: the wait is costing us lives!”
Leena Menghaney, Global IP Advisor for MSF’s Access Campaign:
“On the eve of World TB Day, the rejection of the patent for the salt form of bedaquiline by the Indian Patent Office is welcome news for people with drug-resistant TB in India. We are relieved to see that the Indian Patent Office has once again rejected an evergreening patent application by a pharmaceutical corporation using the public health safeguard enshrined in India’s patent law Section 3(d). This is a seminal decision taken by one of the countries in the world most affected by TB.”
“It is high time that we have alternate manufacturers supplying bedaquiline at lower prices, especially as the scale-up of the all-oral, shorter, six-month drug-resistant TB regimens by TB programmes is being planned around the world. MSF calls on J&J to not enforce this secondary patent in South Africa and other high TB burden countries and stand by its previous commitment to allow generic manufacturers to supply more affordable, quality-assured generic versions of this lifesaving drug.”
Dr Ilaria Motta, TB Medical Advisor for MSF’s Access Campaign:
“We welcome the Indian Patent Office’s decision to reject J&J’s attempt to extend its patent and urge generic manufacturers to use this opportunity to enter the market and produce and supply quality-assured generic versions of the lifesaving TB drug bedaquiline without the fear of litigation hanging over their heads. With more affordable generic versions of bedaquiline likely to become available soon, governments all around the world must act now to roll out the shorter oral regimen for patients with drug-resistant TB, as recommended by the WHO, into their national guidelines so that everyone who needs it has access. In addition, to ensure that more people affected by drug-resistant TB are swiftly and adequately treated, governments should ensure access to early and adequate diagnosis for all people suspected of having TB and drug-resistant TB.”
 Phumeza Tisile, extensively drug-resistant (XDR) TB survivor and petitioner in the patent opposition case. Photographer: Jelle Krings. Date: 14/03/2019. Location: South Africa
Phumeza Tisile, extensively drug-resistant (XDR) TB survivor and petitioner in the patent opposition case. Photographer: Jelle Krings. Date: 14/03/2019. Location: South Africa Candice Sehoma, Access Campaign Advocacy Officer outside Johnson and Johnson offices in Midrand South Africa. Photographer: Boitumelo Zwane. Date: 10/10/2019. Location: South Africa
Candice Sehoma, Access Campaign Advocacy Officer outside Johnson and Johnson offices in Midrand South Africa. Photographer: Boitumelo Zwane. Date: 10/10/2019. Location: South Africa